



版權說明:本文檔由用戶提供并上傳,收益歸屬內容提供方,若內容存在侵權,請進行舉報或認領
文檔簡介
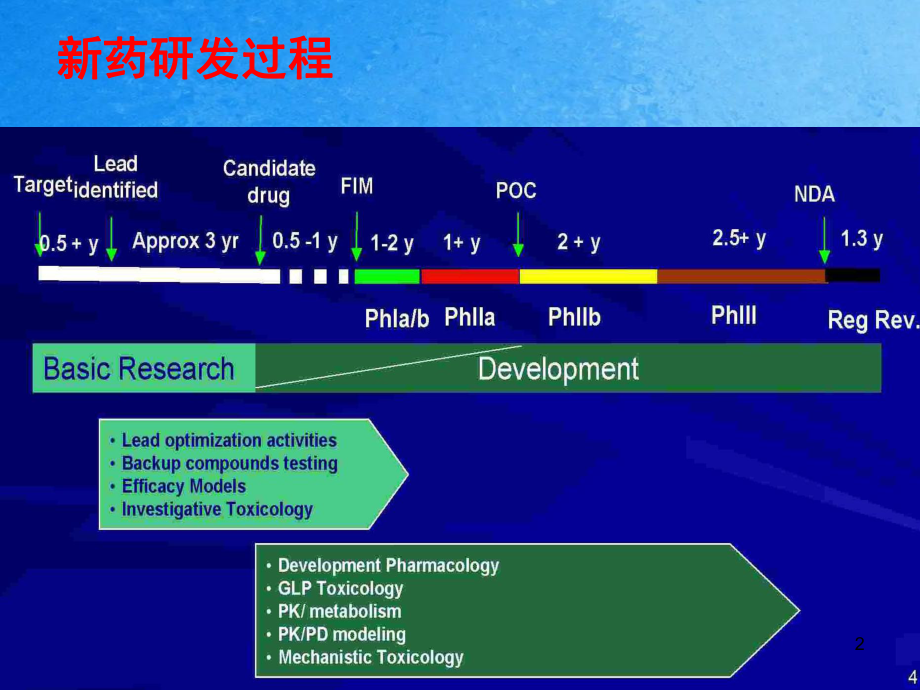
1、1新藥研發過程新藥研發過程2新藥研發過程質量規范新藥研發過程質量規范GLP 歷史沿革歷史沿革 服從服從 GLP 的意義的意義GLP 規范根本要求規范根本要求原始數據核對要點原始數據核對要點 非臨床實驗室常見錯誤非臨床實驗室常見錯誤法規對法規對 GLP 或非或非 GLP的要求的要求案例分析案例分析內容提要內容提要4GLP 歷史歷史 美國美國What prompted US FDA to issue GLP regulations?In the 1960s and 1970s, in addition to the “Thalidomide story, FDA found:Selectively
2、 submitted findingsFabricated dataFalsified dataDiscrepancies in reporting (e.g., between individual and summary data)Poor laboratory recordkeeping (resulting in inability to reconstruct study performance)GLP 歷史歷史 美國美國In the 1960s and 1970s, FDA also found:No protocols, protocols written after study
3、 performance, study not performed according to protocolNo one in charge of studiesSloppy laboratory practicesUS FDA GLP 法規法規1976Congressional hearingsGLPs proposed1978GLPs finalized1979GLPs become effectiveUS FDA GLP 法規法規 21 CFR: Code of Federal Regulations, Food & Drug Administration. 21 CFR Pa
4、rt 58: Good Laboratory Practices for Nonclinical Laboratory Studies 21 CFR Part 11: Electronic Records; Electronic SignaturesUS FDA GLP Part 58 要求要求 Describes requirements for conducting and reporting nonclinical laboratory studies Intent: provides a framework for conducting well-controlled studies
5、assures quality and integrity of the data facilitates study reconstruction provides overall accountability Nonclinical studies that evaluate safety must be GLP compliantUS FDA GLP 檢查檢查 FDA GLP檢查過的美國國內實驗室檢查過的美國國內實驗室200余家余家, CRO, 藥廠藥廠US FDA GLP 檢查檢查 FDA GLP檢查過的美國境外實驗室檢查過的美國境外實驗室40余家余家, CRO, 藥廠藥廠US FDA
6、 GLP 檢查:檢查:MOU 8 個國家個國家 日本日本 法國法國 德國德國 加拿大加拿大 意大利意大利 瑞典瑞典 瑞士瑞士 荷蘭荷蘭US FDA GLP 檢查:中國檢查:中國GLP實驗室實驗室 2021 年年7月檢查了三家月檢查了三家GLP實驗室實驗室 國家安評中心國家安評中心 (NCSED) 昭衍昭衍JOINN) Bridge (康龍化成康龍化成) 昭衍提交的實驗報告獲得美國昭衍提交的實驗報告獲得美國FDA認可認可, 用于支持美國的臨床實驗。用于支持美國的臨床實驗。OECD GLP 規范規范 Developed in 1978 USFDA GLP provided the basis fo
7、r OECD Revised OECD principles adopted in 2019 Primary objective similar to USFDA To ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations in the framework of harmonising testing procedures for the mutual acceptance of d
8、ata (MAD)OECD MAD 數據互認數據互認 Data generated in the testing of chemicals in an OECD member country in accordance with OECD Test Guidelines and OECD Principles of GLP shall be accepted in other Member countries for purposes of assessment and other uses relating to the protection of man and the environme
9、nt OECD Member Countries Australia, Austria, Belgium, Canada, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Japan, Korea, Luxembourg, Mexico, Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Spain, Sweden, Switzerland, Turkey, United
10、 Kingdom, United States中國中國GLP開展歷史開展歷史 1993年年12月,國家科委發布了月,國家科委發布了GLP試行試行 2019年年10月,月,SDA發布發布GLP試行試行 2019年中國修訂年中國修訂、 ,將,將GLP明確為法定要求明確為法定要求 2019年年9月月,SFDA公布實施公布實施GLP二號令二號令280 條條, 開展開展GLP認證檢查認證檢查 2019年年1月月 法規毒理實驗強迫要求法規毒理實驗強迫要求GLP 2021年年7月月 3家在中國的家在中國的GLP 實驗室接受美國實驗室接受美國FDA檢查檢查中國中國GLP管理規范管理規范藥物非臨床研討質量管理規
11、范局令第藥物非臨床研討質量管理規范局令第2號號2019年年9自自2019年年9月月1日起施行日起施行 共九章共九章45條條 非臨床研討質量管理規范認證規范非臨床研討質量管理規范認證規范280條條 藥品注冊現場核對管理規定藥品注冊現場核對管理規定7章、章、59條、條、5個附件個附件服從服從 GLP 的意義的意義 Assures quality data and data integrity Protects the well-being of subjects in clinical trials many of whom are healthy volunteers (human safety
12、) Ensures that a study can be completely reconstructed from archived records 對中國對中國CRO來說,研討報告可以得到國際來說,研討報告可以得到國際認可。認可。GLP 規范要素規范要素What is a nonclinical laboratory? In the SFDA or USFDA regulatory world, a laboratories that conduct nonclinical studies involving test articles to develop data that will
13、 be submitted to the agency in support of an application and marketing approvalGLP 規范要素規范要素Test Articlesthe SFDA-/USFDA-regulated product being testedGLP 規范要素規范要素 Test Systems Mouse/Rat, Guinea Pig, Rabbit, Dog (Beagle), Pig, Monkey, Primate, (Chimpanzee)The ABC of GLP Regulations DefinitionsPersonI
14、ndividualPartnershipGovernment agencyOrganizational unitCorporationScientific or academic establishmentGLP 規范要素規范要素Raw Data Laboratory worksheets Records & document Memoranda Notes Computer print-outs All communications (internal/external/sponsors)GLP 規范要素規范要素Organization and PersonnelEducationT
15、rainingExperienceJob descriptionPersonnel RecordPersonnelManagementStudy DirectorQAUFollow protocolDocument deviationsArchiveGLP 規范要素規范要素Organization and PersonnelReviewQAUPersonnelStudy DirectorMaster schedule sheetProtocolsInspection recordsSOPsManagementStatus reportFinal study reportGLP 規范要素規范要素
16、FacilitiesNonclinical laboratoryReceipt and storageMixingGLP現場核對現場核對Process-oriented quality data as a result of proper utilization of and control over facilities, personnel and proceduresAllows flexibility in laboratory operation and use of scientific judgment study directors must exert this judgme
17、nt overall responsibility for technical conduct, interpretation and reporting現場核對要點現場核對要點Step 1: Inventory Documents 文件清單文件清單Make sure necessary documents were included in the study reportProtocolProtocol amendments 方案修正方案修正Protocol deviations 方案偏離方案偏離(with explanations on possible impact to study i
18、nterpretation and validity)Report (with sufficiently detailed summary and individual animal data)現場核對要點現場核對要點Step 2: Identify test article 供試品供試品test article code or namesalt formformulationpurityUniformity 均一性均一性Stability 穩定性穩定性lot or batch#現場核對要點現場核對要點Step 3: The experimental design 實驗設計實驗設計Studie
19、s are fluid; what is in a protocol frequently changes during the course of a studyNote important dates (experimental design landmarks)experimental or dosing start date (REPORT)study initiation date (REPORT)protocol amendment date(s) (AMENDMENTS)experimental completion date/necropsy date (REPORT)現場核對
20、要點現場核對要點Step 3: The experimental design 實驗設計實驗設計Note how experimental design/methods changedlook at protocol amendments and deviationsconsider if the changes invalidated the studys objectivesconsider if the changes caused study to be inconsistent with stated guidelines/methods (and evaluate signific
21、ance)Make sure you understand experimental design (as performed) and chronology per amendments and documented deviations現場核對要點現場核對要點Step 4: Compare documents for consistency 一致性一致性Do reports comply with GLP requirements on reporting fortest article characteristicstesting of dosing formulations forpu
22、ritystabilityuniformity現場核對要點現場核對要點Step 4: Compare documents for consistency Make sure that data and their associated documents are consistent with one another. 現場核對要點現場核對要點Step 4: Compare documents for consistency Are protocol-specified evaluations of data applied?statistical testscriteria for acce
23、ptable study, positive finding (e.g., genotoxicity tests)Does selection of highest dose comply with protocol? With referenced guideline?現場核對要點現場核對要點Step 4: Compare documents for consistencyAre findings for all protocol-specified evaluations reportedbody weightclinical chemistryhistopathology for all
24、 protocol-specified dose groupstoxicokinetics現場核對要點現場核對要點Step 5: Do data seem credible?Report Raw data/Reality現場核對要點現場核對要點Step 6: Evaluation of significance of lapses 失誤的評價失誤的評價The big questions Did problems in study documents result in your inability to draw meaningful conclusions from the study (w
25、ith respect to studys stated objective)?Did inconsistencies in this part of the submission, relative to other parts of the submission, make you have less faith in the integrity of other portions of the submission? In the conclusions of other studies?現場核對要點現場核對要點Not all lapses preclude drawing conclu
26、sions from a studymistakes can happen in performing a studyin describing fact sometimes fact is not convenient The evaluation of mistakes requires an evalution of their magnitude and nature. Are errors widespread? Limited? Do errors occur in reporting of endpoints that are critical to scientific int
27、erpretation? Do errors speak for a report-specific problem or something that is a systemic problem ?現場核對要點:職責現場核對要點:職責 TESTING FACILITY MANAGEMENT: Overall laboratory management and administrative functionsdesignates study director before study is initiatedreplaces study director (promptly), if nece
28、ssaryassures there is a quality assurance unit (QAU)assures test and control articles have been appropriately evaluated for identity, strength, purity, stability and uniformity (as applicable)現場核對要點:職責現場核對要點:職責TESTING FACILITY MANAGEMENT: (contd)assures appropriate staffing, facilities, equipment an
29、d materials are available for scheduled testsassures that staff understands the functions they are to performassures deviations reported by QAU are promptly reported to study director現場核對要點:職責現場核對要點:職責STUDY DIRECTOR Single point of study controlhas overall responsibility for:Protocol preparationtech
30、nical conduct of studyinterpretation of resultsanalysis of resultsdocumentation of resultsreporting of resultsArchiving現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)Protocol preparation現場核對要點:職責現場核對要點:職責 STUDY DIRECTOR: (contd)Contributors Ophthalmology Cardiology ImmunologyAnalyticalFormulation analysis
31、BioanalyticalStatistical analysisSpecialists/Consultants AntibodiesBone marrow differential countsSpecialized clinical pathologySperm Analysis 現場核對要點:職責現場核對要點:職責 STUDY DIRECTOR: (contd)Special Procedures Considerations Are there SOPs in place? Are the staff appropriately trained? Is this training do
32、cumented? Are literature searches necessary?IACUC implications? Do you need to use a consultant/PI for the work? 現場核對要點:職責現場核對要點:職責 STUDY DIRECTOR: (contd)Multi-Site Studies* Work (i.e. phase of a GLP study) performed at a geographically distinct site (Not a FDA GLP term) Assign a PI to ensure compl
33、iance with GLPsWill sign an Acceptance of Responsibilities formWill sign a statement to this fact upon completion of the work Study director remains the single point of control and maintains responsibility for overall conductQuality assurance of the test site*OECD requirement 現場核對要點:職責現場核對要點:職責 STUD
34、Y DIRECTOR: (contd)Study Scheduling Considerations Test article availability Animal availability/orderingHousingTrained staffAnalytical chemistryClinical pathologyNecropsyReports 現場核對要點:職責現場核對要點:職責 STUDY DIRECTOR: (contd)Test Article Calculations - How much will you need?When is it available?Final d
35、oses?Analytical ConfirmationStorage/handling conditionsCertificate of Analysis (COA), MSDS, purity, stabilityIs there a dose formulation? 現場核對要點:職責現場核對要點:職責 STUDY DIRECTOR: (contd)Protocol Review & ApprovalManagement SDSponsor (if done by CRO) Scientific contributors and laboratory staff Report
36、preparation staffQAUIACUC 現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)Ovetsight of Study Conduct Observe animals and proceduresReview dataCommunicate with scientific contributors and technical staff QA audits internal and externalInteractions with contributors/ PIsSubmission of samplesReceipt/review of
37、 reportRespond to unexpected events現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)Oversight of Study Conduct Protocol amendments - a planned changeProtocol deviations not planned; impact on study must be determinedSOP deviations 現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)Example of DocumentationProtocol/pro
38、tocol amendment Protocol/SOP deviationsAnimal order Test article receipt/information Test article preparation procedureDose accountability (out of range?)Study file notesVeterinary requests/approval of treatmentEnvironmental deviations (e.g. light/dark cycle and humidity) observations of animals/pro
39、cedures Data reviewCorrespondence , fax, letter, telephone callsReports 現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)Report Preparation現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)ArchivingProtocol/amendmentsRaw data DocumentationSpecimensFinal report 現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd)SD Responsibili
40、ties for a Final ReportData interpretationPreliminary draft audited? unaudited?Integration of toxicology, pathology, TK and other supportive dataContributing Scientist/PI reportsStopped/suspended programsGLP compliance Deviations and impact on data 現場核對要點:職責現場核對要點:職責STUDY DIRECTOR: (contd) assures t
41、hat: protocol, including any changes, is approved as specified in GLPs, and is followedall experimental data, including observations of unanticipated responses of the test system, are accurately recorded and verifiedunforeseen circumstances, that may affect the quality and integrity of the study, ar
42、e noted when they occur, and that corrective action is taken and documented現場核對要點:職責現場核對要點:職責QUALITY ASSURANCE UNIT (QAU):Oversees GLP Compliance in laboratoryresponsible for monitoring each study for GLP complianceorganizationally, QAU reports to test facility managementindependent of the personnel
43、 engaged in the direction and conduct of individual studiesassures that facilities, equipment, personnel, methods, practices, records, and controls are in conformance with the GLPs現場核對要點:職責現場核對要點:職責QUALITY ASSURANCE UNIT (QAU): (contd)Oversees GLP compliance in laboratorykeeps up-to-date records of
44、all studies scheduled/performed with master schedule at labmaintains copies of all study protocols現場核對要點:職責現場核對要點:職責QUALITY ASSURANCE UNIT (QAU): (contd) Inspects studiesat intervals adequate to assure the integrity of the studymaintains written and properly signed records at each inspection identif
45、yingdate of inspectionthe study inspectedphase or segment of study inspectedperson performing inspection現場核對要點:職責現場核對要點:職責QUALITY ASSURANCE UNIT (QAU): (contd)maintains written and properly signed records at each inspection identifyingfindings and problems observed during inspectionscheduled date fo
46、r reinspection, if applicableproblems must immediately be brought to attention of study director and management現場核對要點:職責現場核對要點:職責QUALITY ASSURANCE UNIT (QAU): (contd)submits periodic status reports on each study to management and study directornotes problemsnotes corrective actions takendetermines t
47、hat no deviation from approved protocols or standards operating procedures were made without proper authorization and documentation現場核對要點:職責現場核對要點:職責QUALITY ASSURANCE UNIT (QAU): (contd)Reviews final study report to assure that:report accurately describes methods and standard operating proceduresrep
48、orted results accurately reflect the studys raw dataPrepares and signs statements to be included with the final report specifying details on inspections現場核對要點:職責現場核對要點:職責Facility OperationsStandard operating procedures (SOP)Complete and comprehensiveUp to dateSound science and practicalReagents and
49、solutionsIdentity, titer/concentration, storage requirements, and expiration dateAnimal care and IACUCMajor issues of data auditWhat do we look for while auditing a GLP labStudy DirectorFacility ManagementQuality AssuranceChemistryPathology(Clinical and anatomical)Technical StaffReport writingSponso
50、rAccountingSubcontractors非臨床實驗室常見錯誤非臨床實驗室常見錯誤Study directorFailure to follow protocolMost common because everything is driven by protocol. Examples:TA Stability determinationEnvironmental conditionsExposure to test article (dosing)非臨床實驗室常見錯誤非臨床實驗室常見錯誤Study director (contd) Final ReportCommonly see f
51、ailures to address issues that occurred during study that could affect outcomes非臨床實驗室常見錯誤非臨床實驗室常見錯誤Study director (contd) Failure to record all data and verifyFormulationDosing非臨床實驗室常見錯誤非臨床實驗室常見錯誤Study director (contd)Documentation issuesBest way, protocol amendment. Must be done before action (sign
52、ed by SD and also QA, management and sponsor).Second best, deviation report (deviation from protocol or SOP). Completed after-the-fact by person making the observation (signed by SD and also QA and management). Deviation is noted in study report along with description of the impact the deviation has
53、 on study integrity.非臨床實驗室常見錯誤非臨床實驗室常見錯誤 Inconsistencies within a protocol or between protocol and SOP Omission of necessary information from protocol Late entries in study books Non GLP corrections Failure to sign and date entries Expired reagents非臨床實驗室常見錯誤非臨床實驗室常見錯誤 Failure to issue timely protoco
54、l amendments and deviation reports Paperwork missing from study book Inconsistencies between protocol and report or raw data and report.非臨床實驗室常見錯誤非臨床實驗室常見錯誤 QAU fails to authorize deviation Deviations not detected by the QAU, but should have been非臨床實驗室常見錯誤非臨床實驗室常見錯誤Transfer of data, specimens, recor
55、ds to archivesAt completion of studyNot all records transferred非臨床實驗室常見錯誤非臨床實驗室常見錯誤Did not follow SOPs for required auditingInappropriate training record keepingEquipment calibration issuesSanitation cage/room disinfectantsWater system attached to cage rackGLP or not GLP Safety Pharmacology studies,
56、 Core/GLP, follow up studies depending on the design/non GLP Primary Pharmacodynamic/non-GLP, Secondary PD/non GLP unless contribute to the safety evaluation Bridging studies, GLP QT studies, Guidance/GLP, data not required for regulatory submission/ non GLP In Vitro, if pivotal, genotox/GLP, effica
57、cy, MOA, metabolism/non GLPGLP or not GLPStudies that are not within the scope of GLP regulations Include (US domestic only):EfficacyChemical assays for quality controlStability testsConformance pharmacopeia standardsPharmacology and effectivenessNew methodology for toxicology experimentationExplora
58、tory studies on viruses and cell biologyMode of action, synthesis, analysisStudies covered by GMPsGLP or not GLP Disease Model Biologic Systems, Pharmacology, Transgenic animals, efficacy/non GLP, Carc/GLP Animal Rule, Efficacy/GLP Immunotoxicity studies, Guidance does not mention GLP, not pivotal f
59、or safety and most tests routinely not conducted according to GLP Excipients, GLPGLP or not GLPThe Standard is GLPWhen is FDA “more likely to accept non GLP (US domestic only)?Oncology (safety data is from published literature)Biologics (small companies, university, NIH, NCI) tissue cross reactivity
60、 studiesAIDS Drugs (early days, studies done by Academicians)Botanical submissions Known class of drugsOld drugs, change of route of administration to a less hazardous exposure (bridging studies)Drugs marketed overseas, tox studies performed in US but not GLPs (anti malarial, parasitic drugs)GLP or not GLPHowe
溫馨提示
- 1. 本站所有資源如無特殊說明,都需要本地電腦安裝OFFICE2007和PDF閱讀器。圖紙軟件為CAD,CAXA,PROE,UG,SolidWorks等.壓縮文件請下載最新的WinRAR軟件解壓。
- 2. 本站的文檔不包含任何第三方提供的附件圖紙等,如果需要附件,請聯系上傳者。文件的所有權益歸上傳用戶所有。
- 3. 本站RAR壓縮包中若帶圖紙,網頁內容里面會有圖紙預覽,若沒有圖紙預覽就沒有圖紙。
- 4. 未經權益所有人同意不得將文件中的內容挪作商業或盈利用途。
- 5. 人人文庫網僅提供信息存儲空間,僅對用戶上傳內容的表現方式做保護處理,對用戶上傳分享的文檔內容本身不做任何修改或編輯,并不能對任何下載內容負責。
- 6. 下載文件中如有侵權或不適當內容,請與我們聯系,我們立即糾正。
- 7. 本站不保證下載資源的準確性、安全性和完整性, 同時也不承擔用戶因使用這些下載資源對自己和他人造成任何形式的傷害或損失。
最新文檔
- 電機相關主題名稱再次續篇考核試卷
- 灌溉自動化系統在精準灌溉中的應用考核試卷
- 果蔬產品質量分級與包裝規范考核試卷
- 工藝品與收藏品綜合知識競賽考核試卷
- 電子寵物智能穿戴技術考核試卷
- 皮革制品行業的市場渠道與銷售網絡考核試卷
- 文具用品行業環保材料研發與應用考核試卷
- 《垂暮腐朽與閉關鎖國》明清時期課件-1
- 2025屆山西省大學附屬中學高三第一次高考模擬考試數學試題試卷
- 2025一月份智能倉儲系統對接購銷協議技術條款
- 學習通《《詩經》導讀》習題(含答案)
- 2025-2030智能代步車產業市場現狀供需分析及重點企業投資評估規劃分析研究報告
- 《10 水培綠蘿》(教案)-2024-2025學年三年級上冊勞動人教版
- 八項規定試題及答案
- 江蘇省蘇州市2023-2024學年五年級下學期期中綜合測試數學試卷(蘇教版)
- 《思想道德與法治》 課件 第四章 明確價值要求 踐行價值準則
- 一只貓的生命哲學The Zen of Cat(中英文)
- 隧道地表預注漿技術交底(共7頁)
- 通信的知識--家長進課堂(課堂PPT)
- 攪拌站調度室管理制度和獎罰辦法
- 簡易井架方案

評論
0/150
提交評論